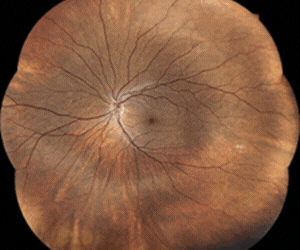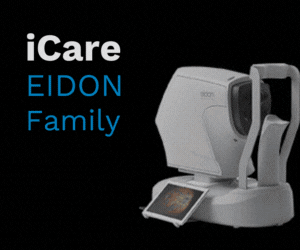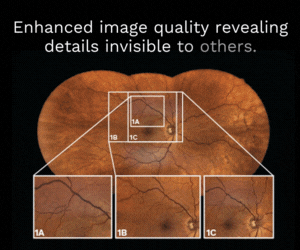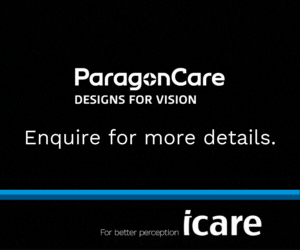
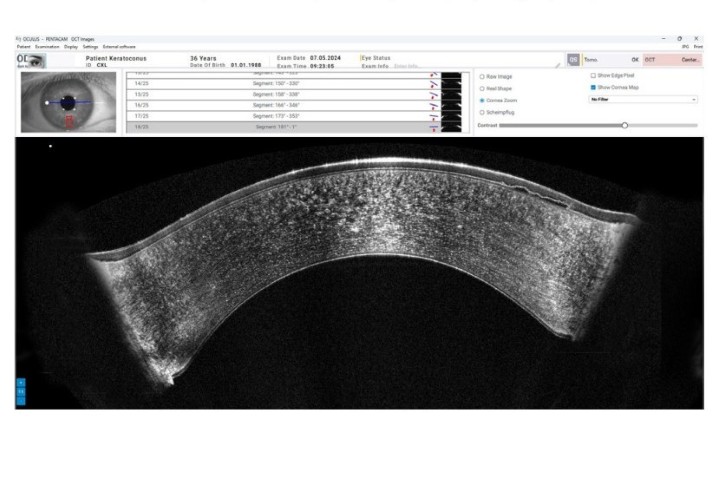
Retinal regeneration to restore sight loss?
Researchers have discovered the retina has a regenerative capacity kept dormant by a cellular signalling mechanism called the Hippo pathway, providing the possibility of one day restoring vision loss by activating the retina’s ability to regenerate.
“Damage to the retina can lead to irreparable loss of vision in humans and other mammals because their retinas do not regenerate,” said lead author Dr Ross Poché, assistant professor of molecular physiology and biophysics at Baylor College of Medicine in Texas. “However, other animals such as zebrafish can reverse blindness thanks to specialised cells in the retina called Müller glial cells. When the retina is damaged, Müller glial cells proliferate and differentiate into the lost retinal neurons, effectively replacing injured cells with fully functional ones.”
Although Müller glial cells in injured mammalian retinas do not restore vision as their counterparts in zebrafish do, other researchers have shown when the mammalian retina is injured, a small subset of Müller glial cells takes the first steps needed to enter the proliferation cycle, such as acquiring molecular markers scientists expect to see in a proliferating cell.
“But this attempt to proliferate is transient. After acquiring some of the cell markers the cells shut off,” said Poché. “These observations suggested the mechanism driving cell repair in zebrafish might be present in mammals, but is actively suppressed. For years, the suppressing mechanism was unknown.”
The researchers focused their attention on the Hippo pathway, a network of molecular events contributing to organ growth during development and the regulation of heart tissue regeneration in response to myocardial infarction. The Hippo pathway has been shown to act like a brake on cardiomyocyte proliferation by inhibiting the activity of another pathway called YAP.
When the researchers genetically engineered Müller glial cells to carry a version of YAP impervious to the inhibitory influence of Hippo, the cells showed major proliferation and acquired a progenitor cell identity. Importantly, a small subset of these Müller glial-derived progenitor cells showed signs of spontaneous differentiation into new retinal neurons.
“Our next step is to develop a strategy to guide proliferating Müller glial cells into differentiation pathways leading to retinal cells capable of restoring vision,” said Poché.