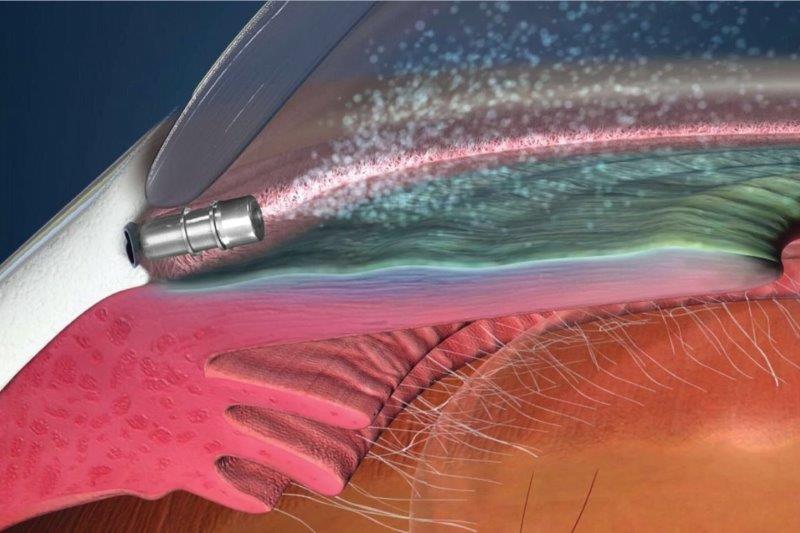
iDose secures FDA approval
Glaukos’ New Drug Application for iDose TR (travoprost 75µg intracameral implant) has been approved by the FDA.
The approval of iDose, a prostaglandin analogue indicated for the reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension, was based on the results from two phase 3 trials, GC-010 and GC-012. These compared the safety and efficacy of a single dose of fast- and slow-release iDose TR to twice-daily topical 0.5% timolol ophthalmic solution in reducing patients’ IOP. The implant achieved mean IOP reductions from baseline over the first three months of 6.6-8.4mmHg in the iDose TR arm, versus 6.5-7.7mmHg in the timolol control arm. At 12 months, 81% of iDose TR subjects were completely free of IOP-lowering topical medications across both trials.
Glaukos said it plans to launch iDose TR in the first quarter of 2024 having set a wholesale acquisition cost of US$13,950 (NZ$22,377) per implant. Well above expectations, US analysts and commentators estimated the price would fall to around US$3,000 to US$5,000 per implant.