New treatments for uveal melanomas and naevi
Targeting ocular malignancies using a novel light-activated virus-like drug conjugate
Maa S, Huis In't Veld RV, de los Pinos E, Ossendorp FA, Jager MJ (2025)
Advances in Ophthalmology Practice and Research 5:49–57
Review: This article explains how a virus-like particle (VLP)-photosensitiser drug conjugate, belzupacap sarotalocan (Bel-sar, previous name AU-011), can be used to treat choroidal melanoma.
Bel-sar is derived from the human papilloma virus and is injected into the suprachoroidal space. It specifically binds to uveal melanoma cells by way of heparan sulfate proteoglycan molecules on the cell surface. The VLP is loaded with a large number of phthalocyanine molecules and, once bound, discharges these into the tumour cells. If bright 689nm light is then shone on the tumour using a laser in the presence of oxygen, these phthalocyanine molecules release singlet oxygen, which is toxic to biological structures. Sufficient damage will kill the tumour cells outright. However, even if they survive this, they will release damage-associated molecular patterns, which the immune system uses to target the tumour cells.
Comment: This novel approach to treating choroidal melanoma offers the advantage of being a highly targeted therapy which, unlike radiation, is less likely to damage surrounding structures. This is particularly important for a tumour close to the macula, which would also get a dose of radiation if the tumour is irradiated by a plaque. And we already have a mechanism to inject it into the suprachoroidal space, as well as a laser that can activate it.
Animal and, more recently, initial clinical trials in the US have proved promising. Now there is a worldwide trial in which both Dr Riyaz Bhikoo and I are involved. Potentially, it offers patients with melanomas close to the fovea a treatment that can kill their tumour without affecting their central vision. Not only that, but there is the possibility that immunogenic cell death (those cells killed by the immune system after being damaged) could trigger a systemic immune response against other tumour cells elsewhere in the body. Thus, this treatment has the potential to kill unrecognised metastatic disease at an early stage.
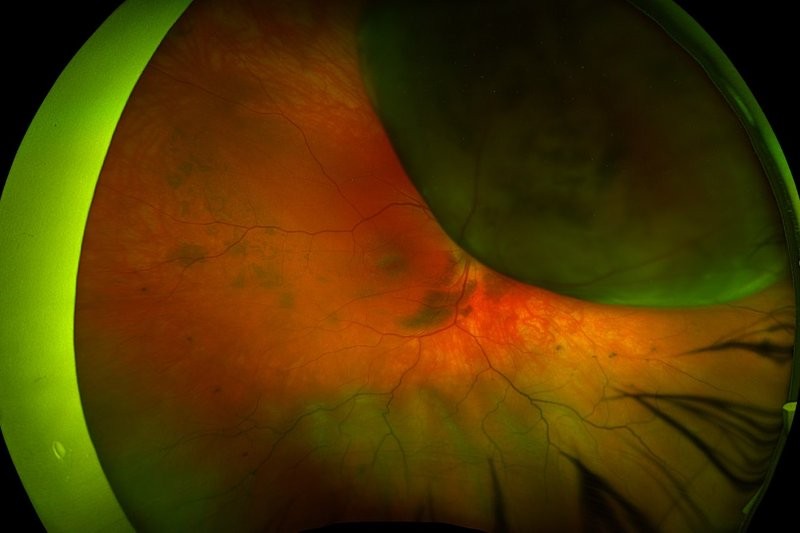
Slow enlargement of choroidal naevi: a long-term follow-up study
Mashayekhi, A, Siu S, Shields CL, Shields JA (2011)
Ophthalmology 118:382–388
Review: This paper is a retrospective observation case series of 278 patients with 284 choroidal naevi who were followed up for at least seven years photographically, without any clinical signs of transformation into melanoma. The median age at presentation was 57 years and the median largest basal diameter of the naevi was 5mm, with a median thickness of 1.5mm. Some had suspicious features, with 6% having lipofuscin and 5% with subretinal fluid extending outside the naevus.
Over a mean follow up of 15 years, 31% enlarged, with the diameter increasing by a median of 1mm (mean, 0.9mm; range, 0.2–3.0mm). The median rate of enlargement was 0.06mm/year (mean, 0.06mm/year; range, 0.01–0.36mm/year). None of the lesions that enlarged developed new risk factors generally associated with malignant transformation. Naevi enlarged more frequently in patients under 40 years (54%) than in those older than 60 years (19%).
Comment: This is not a new study, but it segues into a topic that is prompting research at the moment, mainly due to the advent of widespread fundus photography allowing more patients who have new or growing choroidal naevi to be seen.
It has stimulated unpublished research on the topic of when is a new or growing naevus actually a melanoma. When I attended an International Society of Ocular Oncology meeting in Goa earlier this year, a Danish study (as yet unpublished) was presented showing naevi enlarge and increase in number in the first 40 years of life; in fact, almost no one under 10 years of age had a choroidal naevus. Naevi in that study were present in 2.7% of the population, with 96.6% being common or low risk (0 or 1 on the MOLES scoring system, a gauge of how suspicious naevi are).
Clearly, the vast majority of new and even slowly growing naevi are not melanomas, especially in people younger than 40 years, and this paper give us some handle on how common that is (very common), as well as a rate of change. This is going to become increasingly important to know given that many people have now had their eyes photographed and will have their eyes photographed.
Globe salvage and vision preservation by neoadjuvant darovasertib and crizotinib in uveal melanoma
Hiong A, Roderick O’Day R, Lotte S. Fog LS, Daniel McKay D, McKenzie J, Ameratunga M, Joshua AM, Shackleton M (2024)
Ophthalmology Retina 8: 325-330
Review: This paper, a single case study from Australia, reported the effective use of neoadjuvant darovasertib and crizotinib in a patient with a large uveal melanoma in his only functional eye.
A patient with a blind left eye (secondary to retinal artery occlusion) presented with rapidly declining right eye vision due to a primary uveal melanoma measuring 18mm in maximal diameter and 16.5mm in maximal thickness. This size of tumour is usually treated with enucleation, but such an operation would completely blind him. To salvage vision, neoadjuvant treatment was initiated using darovasertib and crizotinib. After six months of treatment, which included cataract surgery for tumour-associated cataract, the melanoma regressed to 14.1mm in maximal diameter and 2.6mm in maximal thickness, enabling treatment with plaque brachytherapy.
The authors concluded this combination of darovasertib and crizotinib for uveal melanoma is an effective neoadjuvant strategy warranting further investigation as an approach to improve visual outcomes in the treatment of primary uveal melanoma.
Comment: The treatment of metastatic uveal melanoma remains unsatisfactory, but in recent years we have started to learn about the mutations these tumours carry and sought drugs which might target cells with those mutations.
Emerging treatments for uveal melanoma include those targeting mutations in GNAQ and GNA11, which encode members of the G-protein alpha subunit, Gq and G11 and are present in >90% of these tumours. GNAQ/11 mutations lead to activation of protein kinase C (PKC), resulting in activation of the Raf-MEK-ERK pathway and promotion of cell proliferation. Inhibition of PKC has been shown to suppress the growth of GNAQ/11-mutant melanoma cells and has activity in patients with uveal melanoma.
However, targeting PKC alone doesn’t seem to be enough. A potential mediator of resistance to Raf-MEK-ERK inhibition in uveal melanomas is hepatocyte growth factor, which binds to c-MET (mesenchymal-epithelial transition factor), a receptor tyrosine kinase, leading to increased cell proliferation. In uveal melanoma cells, hepatocyte growth factor blunts the effect of MEK inhibition on cell growth, but this resistance can be overcome in vitro using c-MET inhibition, giving rise to the rationale of combining darovasertib, a selective PKC inhibitor, with crizotinib, a c-MET inhibitor. This combination has been evaluated in metastatic uveal melanoma, with early clinical data demonstrating disease control and response rates of approximately 90% and 45%, respectively.
The authors took this potentially promising treatment and, in an apparent act of desperation, tried to shrink a tumour which, given its size, would have ordinarily required enucleating his only eye… and it worked!
However, there are a few potential problems. One is that these drugs are pretty toxic and have a lot of potential side effects. Another is that, having been effective in this patient, it was tried in others, for whom it didn’t work so well. Finally, does it kill all the cells? The aim is to sufficiently shrink a tumour to allow treatment with a plaque instead of having to remove the eye. So, in the end the plaque is a smaller diameter than the original tumour. However, could there be viable cells outside the area that you plaque that you can’t see? And could these cells start growing again and the tumour come back?

Dr Peter Hadden is an ophthalmologist based at Eye Institute Remuera and New Lynn, as well as Greenlane Clinical Centre. He specialises in cataract and vitreoretinal surgery, as well as ocular oncology.