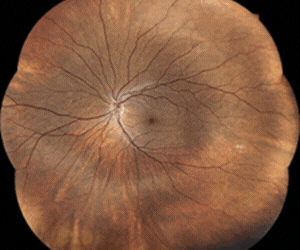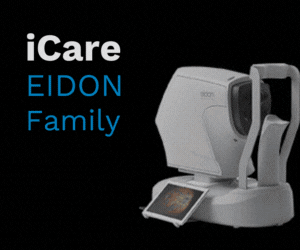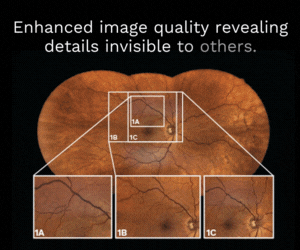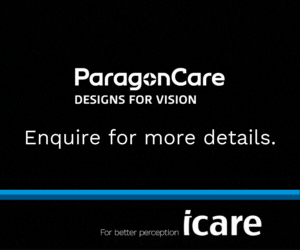
Presbyopia drop draws closer
Allergan has released early Phase III clinical trial results demonstrating the safety and efficacy of its investigational eye drop for presbyopia treatment.
“The positive results from the Gemini studies show the potential of this investigational optimised formulation of pilocarpine 1.25% to treat symptoms associated with presbyopia,” said Dr Michael Robinson, vice president of Allergan’s parent company AbbVie.
The topical AGN-190584 drop, a cholinergic muscarinic receptor agonist, is delivered once daily using a proprietary vehicle. According to AbbVie, it works through dynamic pupil modulation, an effect in which the iris sphincter is contracted to increase depth of focus, and enables increased accommodation through mild contraction of the ciliary muscle.
The identical Gemini 1 and 2 trials enrolled a total of 750 patients, randomised for a one-to-one ratio of placebo to AGN-190584 (pilocarpine 1.25%). AGN-190584 was administered bilaterally for 30 days.
The primary endpoint (the proportion of patients gaining three lines or more on a reading chart in low light) was met in both trials. The majority of secondary endpoints were also met in both studies, including a significant improvement in patient-reported outcomes. No serious adverse events were observed, while the most common non-serious adverse events (occurring at a frequency of ≥3% in AGN-190584-treated participants) were headache, conjunctival hyperaemia, blurred vision and eye pain.
The study results will form the basis for Allergan’s drug application submission to the US Food and Drug Administration in the first half of 2021 and will be presented in greater detail at future medical meetings.
For more on presbyopia eye drops, see https://eyeonoptics.co.nz/articles/archive/presbyopia-eye-drops-the-next-big-thing/