Retina research review: DR, GA and PAMM
Characteristics of participants in DR clinical research clinical trials who were lost to follow-up
Bowe T et al
Retina. 2024 Jan 1;44(1):111-116
Review: The aim of this retrospective cohort study was to delve into the racial/ethnic composition, health status and disease severity of participants enrolled in the Diabetic Retinopathy Clinical Research Network (DRCR) trials who were lost to follow-up. Eight clinical trials of 3,492 participants meeting the inclusion criteria were analysed. The study revealed the characteristics associated with non-completion were younger, predominantly Hispanic or Black ethnicity and type 1 diabetes mellitus. Additionally, this subgroup displayed worse baseline best-corrected visual acuity, higher haemoglobin A1c levels, elevated blood pressure and proliferative diabetic retinopathy (PDR) with a diabetic retinopathy severity score exceeding 66.
This study emphasises the limitation in clinical trials to present a diverse demographic and health-related factors in large populations and the challenges of retaining patients with worse health status.
Comments: Up to 38% of diabetic patients are lost to follow-up, which is particularly problematic in patients with comorbidities and specific collectives. In New Zealand, we see high-risk vision-loss conditions in patients, especially Māori and Pasifika, who face more significant barriers to accessing the healthcare system. Our public healthcare system faces challenges in delivering quality eyecare to the entire community, disproportionally impacting certain patient groups. Engaging individuals and communities at higher risk is critical to improving outcomes in at-risk patients.
Complement inhibition for GA – a review of salient functional outcomes and perspective
Spaide, Richard et al
Retina 43(7):p 1064-1069, July 2023
Review: The aim of this article was to assess the rationale and outcomes of randomised trial results for complement inhibition in the context of geographic atrophy (GA). The study focused on data from recently completed trials, specifically examining pegcetacoplan and avacincaptad pegol and analysing outcomes related to the area of autofluorescence loss and functional vision tests. The results revealed pegcetacoplan, at a 2mg dose, demonstrated a statistically significant reduction in the expansion of autofluorescence loss in a 12-month phase two trial with monthly dosing. However, nearly 40% of patients in the monthly arm did not complete the treatment. In phase 3 studies, there was a statistically significant reduction in the area of atrophy in one of the studies, compared to untreated controls. Avacincaptad pegol also showed a significant reduction in autofluorescence loss at 12 months but did not exhibit benefits in functional outcomes.
Interestingly, each drug increased the risk of macular neovascularisation. While both avacincaptad pegol and pegcetacoplan demonstrated differences compared to sham in autofluorescence imaging, there was no discernible benefit in visual function at 12 and 24 months, respectively.
Comments: As ophthalmologists, we are very excited about treatments for GA. After lampalizumab, the results of avacincaptad pegol and pegcetacoplan must be assessed in the right context and explained properly. The difficulties of these new treatments in Europe are a challenge which we hope their developers will address. A key point is managing the expectations of patients with GA and their options. Unfortunately, it is unlikely that any treatment will improve vision, so decreasing the progression rate of the disease is the most realistic expectation. It will be critical to have longer time analysis and more stringent selection criteria to maximise benefit.
Detection of PAMM can prevent blindness and death
Bousquet, Elodie et al
Retina 43(11):p 1827-1832, November 2023
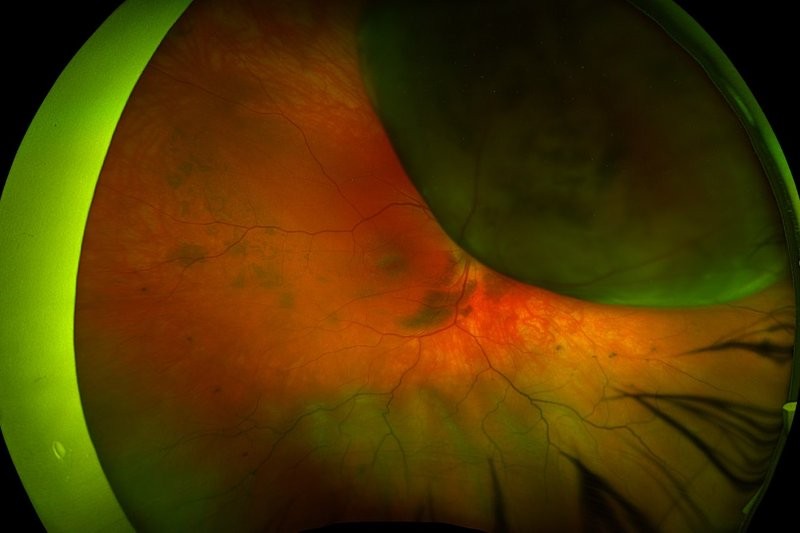
Review: The aim of this study was to review paracentral acute middle maculopathy (PAMM). PAMM is a paracentral hyperreflective band in OCT at the middle retina level, indicating infarction of the inner nuclear layer (INL). It is often linked to a permanent paracentral scotoma. Initially, it was described as associated with central retinal vein occlusion (CRVO) corresponding to previously identified perivenular deep white lesions seen in the fundus. Cross-sectional OCT and adaptive optics imaging later confirmed the localisation of these lesions to the middle retina.
PAMM leaves behind a legacy of INL thinning, recognised as a retinal ischaemic perivascular lesion, detectable as a focal area of irregularity on OCT. PAMM primarily develops due to impaired perfusion of the deep retinal vascular complex, particularly the deep retinal capillary plexus (DCP). It represents the mildest form of macular ischaemia in eyes with CRVO and central retinal artery occlusion. The ischaemic cascade, reflecting the progression of ischaemia with time and severity, supports the predominant vertical organisation of the retinal capillary plexus, emphasising the vulnerability of the DCP to ischaemic injury.
PAMM can result from various aetiologies, with retinal vascular occlusions, especially CRVO, being the most common; it may also serve as a crucial indicator of potentially severe or life-threatening diseases such as giant cell arteritis (GCA) or carotid occlusive disease. Hence, recognising PAMM promptly is of utmost importance as it necessitates an urgent referral for stroke evaluation. This not only helps rule out cardiovascular disease but also excludes GCA in elderly patients, potentially offering remarkable benefits to the patient, in terms of early intervention and appropriate medical care.
Comments: PAMM implications in ischaemia and general health status are fascinating. It is important to take note of small details during an eye examination that can easily go unnoticed. We see plenty of cases of PAMM in relation to Covid-19, but other cases can be a manifestation of advanced and high-risk cardiovascular comorbidities.

Dr Francesc March de Ribot is an ophthalmologist at Dunedin Hospital and a member of the research team at the University of Otago.