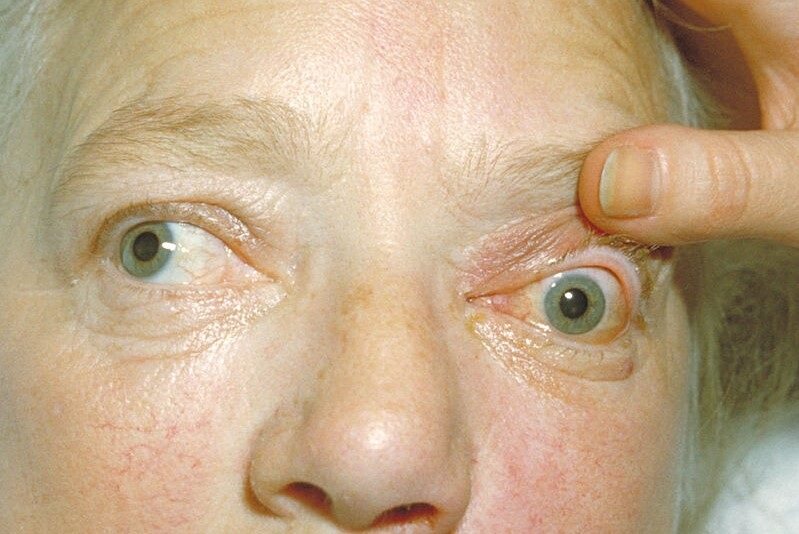
TED treatment trial success
Innovent Biologics announced its phase 3 registrational study (Restore-1) of IBI311, a recombinant anti-insulin-like growth factor 1 receptor antibody, in Chinese subjects with thyroid eye disease (TED) achieved its primary endpoint.
At week 24, 85.8% of subjects treated with IBI311 showed a reduction in proptosis of ≥2mm from baseline in the study eye without a corresponding increase of proptosis in the fellow eye, compared to 3.8% of those receiving placebo. The secondary endpoints of percentage of subjects with a clinical activity score of 0 or 1 and mean change in proptosis from baseline in the study eye were also met.
There are currently no targeted drugs approved for TED in China and the treatment costs of overseas targeted drugs are beyond many patients’ reach, said Dr Lei Qian, Innovent’s vice president of clinical development. “IBI311 has demonstrated significant efficacy and favourable safety in the treatment of TED in the Restore-1 study. We plan to submit its NDA (new drug application) as soon as possible and bring high-quality, effective and safe biological drugs to Chinese patients with TED.”